Reaction of Cyclohexene With Bromine
Bromine does not react with cyclohexane without UV light with or without water. Intramolecular carbonyl ene reaction.

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
Reports of enantioselective reactivity retain this limited substrate scope and are characterized by poor catalytic efficiency that can result in multiday reaction times 26 27.
. Esteves Synthesis 2010 2379-2382. In the second step of the mechanism both H 2 O solvent and Br produced in the first step are nucleophiles and have the chance to react with the cyclic bromonium ion. Treating cyclohexene with 11-diiodoethane and a zinc-copper couple leads to tw- someric products A.
Figure 104e Formation of Halohydrin Figure 104f Mechanism. Explain the formation of major and minor products by reference to the relative stabilities of primary secondary and tertiary carbocation intermediates. When you write the mechanisms involving sulphuric acid keep that shaded part unchanged throughout - apart from where you would change the bromine.
Want to see the full answer. Three alkynes goes under a 222 cyclization reaction and rapidly join to form a. The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable reactions of cyclohexene are shown in the green box.
The formation of major and minor products in addition reactions of unsymmetrical alkenes. Students should be able to. Cyclohexene epoxide opening axial.
Citation neededHowever the International Union of. Stereoselectivity 2182 Kinetic resolution 96 Absolute configuration 90 Stereospecificity 11 Regiochemistry 7 Functional. Chelated reduction zinc borohydride.
In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or. Diels-Alder reaction will occur that forms a cyclohexene. Check out a sample.
Such reaction is highly selective in stereochemistry. A synthetically useful radical-relay CH oxidation. Chelate Cram Addition MeMgBr.
However since H 2 O is the solvent its concentration is much higher than that of Br so the major. Aldol Reaction syn product. ZIF Zeolitic Imidazolate Framework is a metal organic framework MOF made by coordinated by four in the same way as Si and Al atoms are covalently joined by bridging oxygens in zeolitesThe sphere represents the pore size within the.
Science Chemistry QA Library Consider the reaction of bromine with the pictured alkene. Our modern society is based to a large degree on the chemicals we discuss in this chapter. The majority of examples feature only simple cyclic alkene substrates such as cyclopentene and cyclohexene.
Colour of the bromine water solution is decolourized as it reacts with ethene. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. But liquid alkenes like cyclohexene react with bromine water solution in the.
Most are made from petroleum. What happens to that unit is exactly the same as happens to the bromine in the reaction involving HBr. The reaction takes place at room temperature if the reactants are in the gaseous state ethene.
In organic chemistry an alkene is a hydrocarbon containing a carboncarbon double bond. Stereoselective Aldol Reaction Cis gives Syn. Alkynes under metal catalysts for example cobalt can also go under cycloaddition reaction called alkyne trimerization.
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. Outline the mechanisms for these reactions. Consider the reaction of bromine with the pictured alkene.
The reaction between cyclohexene with diiodoethane in zinc. The ICSC project is a common undertaking between the World. Heck reaction 52 Trifluoromethylation 37 Claisen rearrangement 34 Solvolysis 33 Transesterification 30 Dehalogenation 20 Deamidation 4 Grignard reaction 2 Diels-Alder reaction 1 Stereochemistry.
The reaction is regioselective leading to Markovnikov-oriented products and the halofluorinated adducts follow anti-addition in the case of cyclohexene and 1-methylcyclohexene. This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents. A demonstration of bromine substitution and addition reactions is helpful at this point.
Alkene is often used as synonym of olefin that is any hydrocarbon containing one or more double bonds. Felkin-Anh reduction LiAlH 4. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Reaction of bromine water with cyclohexene. Two general types of monoalkenes are distinguished. When the sulphuric acid reacts the whole of the shaded part of the molecule remains as a complete unit.
Also called α-olefins terminal alkenes are more useful. A thought occurs to me. The reaction equation for bromine addition of ethene for example is.
You are not reacting bromine with cyclohexane you are DISSOLVING bromine in cyclohexane and water mixed. The main target users are workers and those responsible for occupational safety and health. The use of bromine to test for unsaturation.
Consider the reaction of bromine with the pictured alkene. Aldol Reaction anti product.
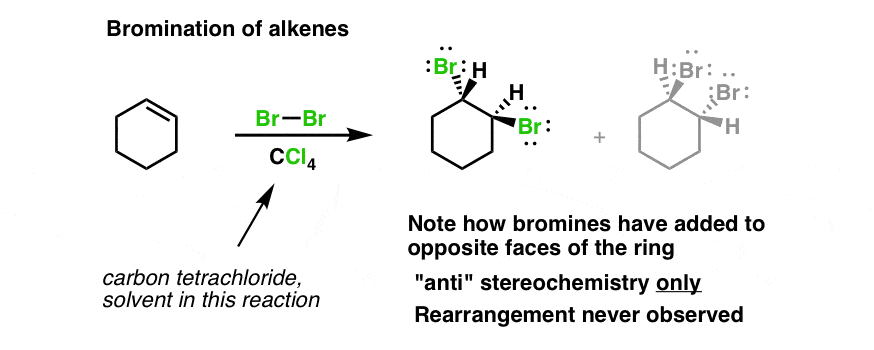
Bromination Of Alkenes Master Organic Chemistry
What Is The Equation Of The Reactions Between Cyclohexene With Br2 Ccl4 What Type Of Reaction Is This Quora
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Bromination Of Cyclohexene Under Conditions Given Below Yields Ltimg Src Https D10lpgp6xz60nq Youtube
Comments
Post a Comment